by Dennis Crouch
In a decision that reinforces the controlling force of explicit patent definitions, the Federal Circuit affirmed a district court’s claim construction that effectively ended Alnylam Pharmaceuticals’ patent infringement suit against Moderna over lipid nanoparticle technology used in COVID-19 vaccines. Alnylam Pharmaceuticals, Inc. v. Moderna, Inc., No. 2023-2357 (Fed. Cir. June 4, 2025). Writing for a unanimous panel, Judge Taranto explained that when a patent specification clearly defines a claim term, the patentee faces a demanding standard to establish exceptions to that definition, even where the definition might exclude disclosed embodiments or seemingly conflict with other parts of the specification.
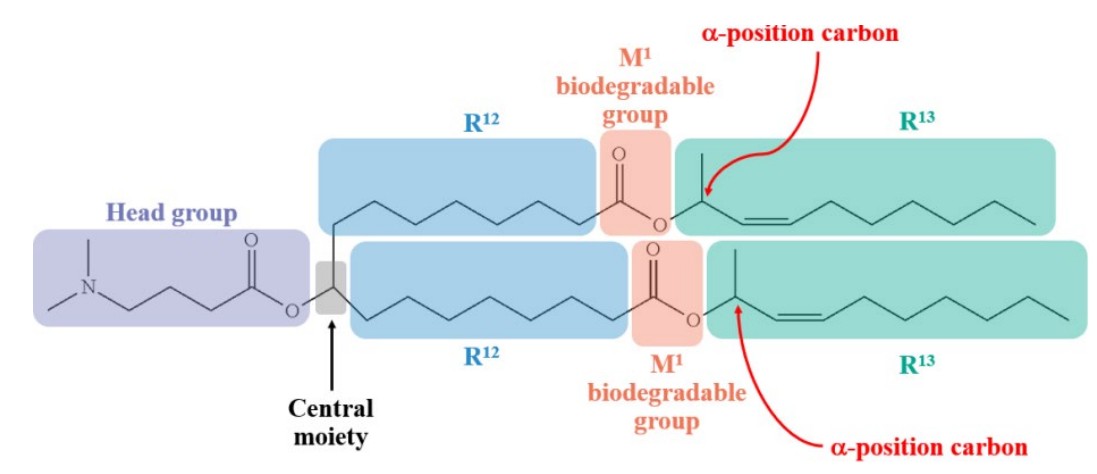
The case centered on whether Alnylam’s patents covering cationic lipids for nucleic acid delivery were infringed by Moderna’s use of the SM-102 lipid in its SPIKEVAX COVID-19 vaccine. The dispute turned entirely on claim construction of the term “branched alkyl,” which the specification explicitly defined as requiring “one carbon atom in the group (1) is bound to at least three other carbon atoms and (2) is not a ring atom of a cyclic group” “[u]nless otherwise specified.”


Alnylam argued this definition did not apply to claims covering “branching” at the “alpha position” adjacent to biodegradable groups, where the chemical structure would permit only two carbon-carbon bonds rather than three. The Federal Circuit rejected this argument, finding no clear indication in the intrinsic record that the claims “otherwise specified” a departure from the explicit definition.

#Strict #Standard #Overriding #Patent #Lexicography #COVID #Vaccine #Patent #Battle